VIP member
UDI, UDI solution, UDI supplier, UDI system, UDI software, high coding, unique identifier for medical devices
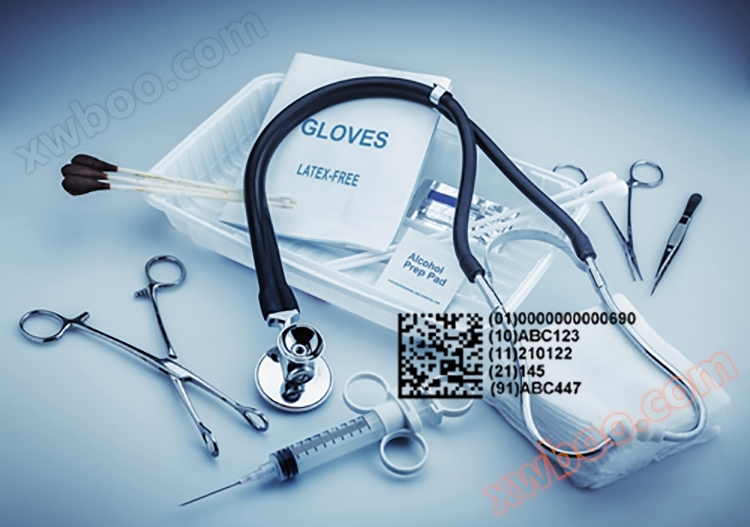
Unique identification of medical devices is a code consisting of numbers, letters, or symbols attached to medical device products or packaging, used t
Product details


The unique identification of medical devices is a type of label attached to medical device products or packagingA code composed of numbers, letters, or symbols used to uniquely identify medical devices.

UDI Solution
Gaofu Code is the preferred partner for many pharmaceuticals, medical devices, and automated production linesCompanion, provide comprehensive solutions to meet high-speed serialization requirements, including UDI.
•Thermal transfer printer, automatic printing and labeling machine, laser coding machine, inkjet printer, high-speed readerCoderIt is a perfect solution for achieving unique identification of the smallest packaging at the first level;
•Labeling machine and large character outer box coding machineThis is the ideal choice for secondary outer box labeling;
•Printing and labeling machineCan simultaneously meet the requirements of automated printing and labeling for secondary outer boxes and tertiary palletizing.
A professional sales team provides professional consultation and solutions for customers' UDI needs,High quality service ensures quick support and response.



1. Boxed and aluminum-plastic foam cover:
CO2 laser coding machine or inkjet coding machine, by burning the coating to mark UDI information, permanent identification is helpful for later tracking and tracing needs;


2. Medical devices made of metal materials:
Fiber laser machines are skilled in engraving, color changing labels, and products made of metal materials such as stainless steel. They are widely praised for their high precision and are fully utilized in metal materials. The UDI numbers are clear and distinct, and the QR code scanning machine can read them with extremely high reading rates.



3. Identification of various medical device labels:
German CAB series heat transfer printer, 600dpi high-quality printing, comparable to printing effect; Avoid barcode distortion, displacement, blurring, and other issues that may occur with similar products.

4. Identification of various medical device labels:
High coding digital color printer, 1200dpi color printing, solves the problems of customized color labels, limited quantity, and multiple styles, helping enterprises efficiently sample and ship.

Fully automatic real-time packaging and labeling solution



5. Identification of various medical device labels:
High coding online printing and labeling, with rich interfaces and built-in IO interfaces; Accurate peeling and precise labeling; There are various labeling methods.


6. Packaging for various medical devices:
High coding online printing and labeling, 300-600dpi printing, comparable to printing effects; In addition to consistent clarity and readability, different labeling schemes are provided for different labeling methods.
Labeling scheme: front labeling, side labeling, rolling labeling, blowing labeling, corner labeling, etc.


Fully automatic real-time packaging and labeling solution


6. Barcode level detection:
Comparison of tag content
Product printing content verification, wide reading content
OCR character recognition
Template comparison, barcode level detection
Database comparison, including variable data comparison
Barcode reading barcode 128A, B, C code EAN-8/13、 39 code, GS1-128Data Matrix QR code, interleaved 25 code UPC-A/E……
One dimensional barcode defect inspection
Multiple areas can be checked simultaneously

Benefits brought by UDI implementation:
After equipping with UDI identification scheme, enterprises can distinguish products of different models and specifications produced by various factories;
• Able to obtain partial information related to medical device registration;
• Able to track and trace in the global supply chain, achieve fast retrieval, thereby helping to reduce medical errors and integrate simplified data systems;
• Promote the rapid identification of adverse events in medical devices, the rapid promotion of problem reporting solutions, and the rapid and effective implementation of device recall plans.




Online inquiry